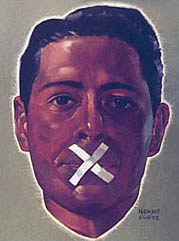
This blog is about the distortion of scientific debate, most particularly by
powerful forces in
medicine. It is about the way in which
industry,
professional bodies,
government regulators and powerful individuals collude to prevent scientific debate and to victimize those asking difficult questions (
www.nhsexposed.com). It is about the way those entrusted with authority behave.
I have been contacted by many individuals who have found themselves in difficulty. Some of these stories are urgent enough for me to want take a break from my most interesting correspondence with
Dr Larry Games Vice President at Procter & Gamble Pharmaceuticals.

One such case is that of the
psychologist Lisa Blakemore Brown, a specialist in Autism, ADHD & Aspergers [
website] [
Book]. Blakemore Brown has been involved on the "wrong side" of the
debate about the psychiatric disorder Munchausen syndrome by proxy (MSbP), maintaining that many parents have been falsely accused of injuring their children. There have been high-profile releases from jail of women such as
Angela Canning. MSbP is a disorder in which an adult invents or deliberately creates a child’s illness to draw attention to themselves. She has challenged prominent doctors such as Sir Roy Meadow and Professor David Southall who, in her view, have promulgated a wholly inappropriate approach to scientific evidence. She has irritated pharmaceutical companies. But instead of debate Lisa has encountered its very opposite. The abuse of science goes right into the heart of a prominent professional body. Her colleagues have stood by in silence.
I have no special knowledge of the science that underpins the debate surrounding autism, MSbP or vaccine side effects. But I do know that debate is important. It is the lifeblood of science. I will be discussing much more of this tragic case over the next few weeks. It is not only a tragedy for Blakemore Brown, but also part of the tragedy of medicine.For now I simply place in the public domain a letter written this week by John Stone and myself to the British Psychological Society. It speaks for itself.
Ray Miller, President,
The British Psychological Society
St Andrews House
48 Princess Road East
Leicester LE1 7DR 14 January 2007
Re: Lisa Blakemore Brown
Dear Mr Miller,
We are writing to express our concern regarding the treatment of Lisa Blakemore-Brown (LBB) by the British Psychological Society. The actions of the Society are such as to cast serious doubts upon its motives as well as upon its plausibility as a professional regulatory body.
It is disturbing that the Society appears to be acting to suppress open debate about controversial theories. Our purpose here is not to get involved in this debate, nor do we necessarily agree with her views. Ms Blakemore-Brown's views are in fact irrelevant. She is entitled to hold any views and to express these, no matter how uncomfortable they are to yourselves. This is enshrined by Article 10 of the Human Rights Act 1998. It seems that the Society have developed an unhealthy obsession with preventing free speech through abuse of mental health diagnosis. Its actions may also be construed as a breach of the Harassment Act 1997.
It cannot be in the interests of society, human rights, patients and of the British Psychological Society to suppress open debate and academic freedom through such mechanisms. The society seems to have encouraged an endless series of unsupportable complaints against LBB, and then progressed them despite evidence that they were not sustainable. The society itself then generated an entirely different complaint (about her irritated response to these very complaints). This is not a proper example for resolving scientific or academic disputes. It appears to be more a method of silencing a critic.
Irritation with a professional body is not in any event an offence. Neither is annoying a professional body. Disagreement with the professional "view" is not a reason to refer an individual for psychiatric assessment except in a Stalinist state. This approach of the BPS is wholly anti-academic and unprofessional. To quote Kingsley Amis "If you can't annoy someone, there's little point in writing". It is also not a prime facie offence to perceive oneself to have been bullied, as the BPS seem to be suggesting.
Having read the case transcripts, we must confess that we find them most extraordinary. The transcript of the first three days of the Fitness to Practice hearing July 2006 reads like an encyclopaedia of legal and psychological abuse. If LBB has responded with irritation, this would seem to be understandable.
- Lisa had been coerced into "hearings" despite having left the society years before. The main charge was modified progressively until it bore no relation to the flawed original charge. The modified "charge" of supposed mental illness (so called "paranoia") was not revealed to Lisa for months after the process had been set in motion.
- Evidence was assembled by the panel as if having been provided by Lisa herself, and presented to others in a jumbled order and without context to suggest mental incoherence in her correspondence with the BPS (a supposed offence).
- In one instance it emerged that the material was forged. Despite that, the original complainants were not invited to be cross-examined, and no action was taken against them after the information was dropped.
- An independent psychiatric report declaring LBB perfectly lucid, quite normal and fit to practice was rejected, and others were requested instead. This is a rather interesting approach for a "psychological society" towards the reliability of such reports. This interesting approach of the BPS appears to be on the basis of the findings of the reports themselves rather than upon the methodology used (since the panel seemed quite happy to consider an assessment based only on LBB's correspondence with the BPS complaining about her treatment, compiled without seeing "the patient" and without any relevance whatever to her clinical practice). More convincing evidence supporting justifiable paranoia and predetermination would be hard to find.
- A psychiatrist declared Lisa to be unfit to practice with the diagnosis of "paranoia" without examining her, and on the basis of material constructively assembled by the committee. Having read the transcript relating to this material we find this "diagnosis" intriguing, and wonder whether a majority (or even any) other psychiatrists or members of the public would reach such a conclusion based on the same information if we were to provide it to them. In any event the material bears no apparent relation to her practice, only to her views about the suppression of scientific debate.
The society has acted callously over a sustained period seeking to undermine and silence Ms Blakemore Brown, despite her unfortunate family circumstances. It has used the practice of psychiatry and psychological assessment in a non-evidence-based way as a tool for destruction. It cannot improve the reputation of the society to be seen to act in such an arbitrary way using its own tools of trade.
The society must bring this charade to an end before any more damage is done, both to society itself and to the chances of proper public discourse in an atmosphere that is free from fear.
- We would appreciate the views of the society before taking this matter forward in terms of public discussion.
- We are unable to find any list of the psychological traits that would render an individual unfit to practice and would appreciate a copy of the same. If supposed "paranoia" or "irritation with the BPS" is on such a list, perhaps bullying should also be added.
- In addition we would also request that the society provide what scientific evidence it has in relation to the, indications for psychiatric assessment in such cases, as well as the reproducibility and plausibility of such reports.
- So bizarre are the case transcripts, we believe that open discussion is required. We intend to publish these in full, with appropriate commentary as part of a campaign to prevent such behaviour by professional regulatory bodies. If the society can see any reason such publication should not take place, we would appreciate it if you would let us know those reasons.
Yours sincerely,
Mr John Stone
Dr Aubrey Blumsohn
Please contact me if you want to help (
Email me)
On 25 January 2007 we received the following "reply" addressing none of the issues raised. The discussion with regard to confidentiality was particularly surprising in that the BPS has since threatened legal action if Blakemore-Brown publishes the transcript.
[Address]
I am surprised that you have seen transcripts of proceedings which are confidential for the benefit of the respondent to those proceedings, and which the Society has neither intention nor authority to publish.
This matter has yet to be concluded, and consequently no comment can be made on it.
I would remind you that the transcripts belong to the Society and are confidential to Ms Blakemore-Brown and any publication would be entirely inappropriate and a breach of confidence.
Yours sincerely
RAY MILLER
President, British Psychological Society
Earlier|
Later|
Main Page
 From: Aubrey Blumsohn
From: Aubrey Blumsohn One such case is that of the
One such case is that of the  These issues go to the heart of science and the responsibility of scientific authors. His reply and my reply to his reply are below. Dr Games has promised to write back again to explain what Procter and Gamble mean by "access to data" given repeated refusal to provide it to authors (see some part of that
These issues go to the heart of science and the responsibility of scientific authors. His reply and my reply to his reply are below. Dr Games has promised to write back again to explain what Procter and Gamble mean by "access to data" given repeated refusal to provide it to authors (see some part of that 